Statin Cramp Diagnostic Tool
Symptom Assessment
Select your symptoms to determine if you're experiencing statin-induced myopathy or neuropathy.
When you hear the term “statin cramps,” the first thought is usually sore legs after a run. In reality, the aches can signal two very different problems - one rooted in muscle, the other in nerves. Figuring out whether you’re dealing with statin myopathy or a statin‑related neuropathy is crucial because the treatment paths diverge sharply.
What Are Statins and Why Do They Cause Cramps?
Statins are a class of HMG‑CoA reductase inhibitors that lower LDL cholesterol and reduce cardiovascular risk. Since lovastatin hit the market in 1987, millions have taken them to keep heart attacks at bay. Yet, 7‑29 % of users report muscle‑related symptoms, a group researchers call statin‑associated muscle symptoms (SAMS). The exact mechanism isn’t a single culprit; reduced coenzyme Q10, disturbed mitochondrial function, and altered protein prenylation all play a role.
Statin‑Associated Myopathy: The Muscle‑Side Story
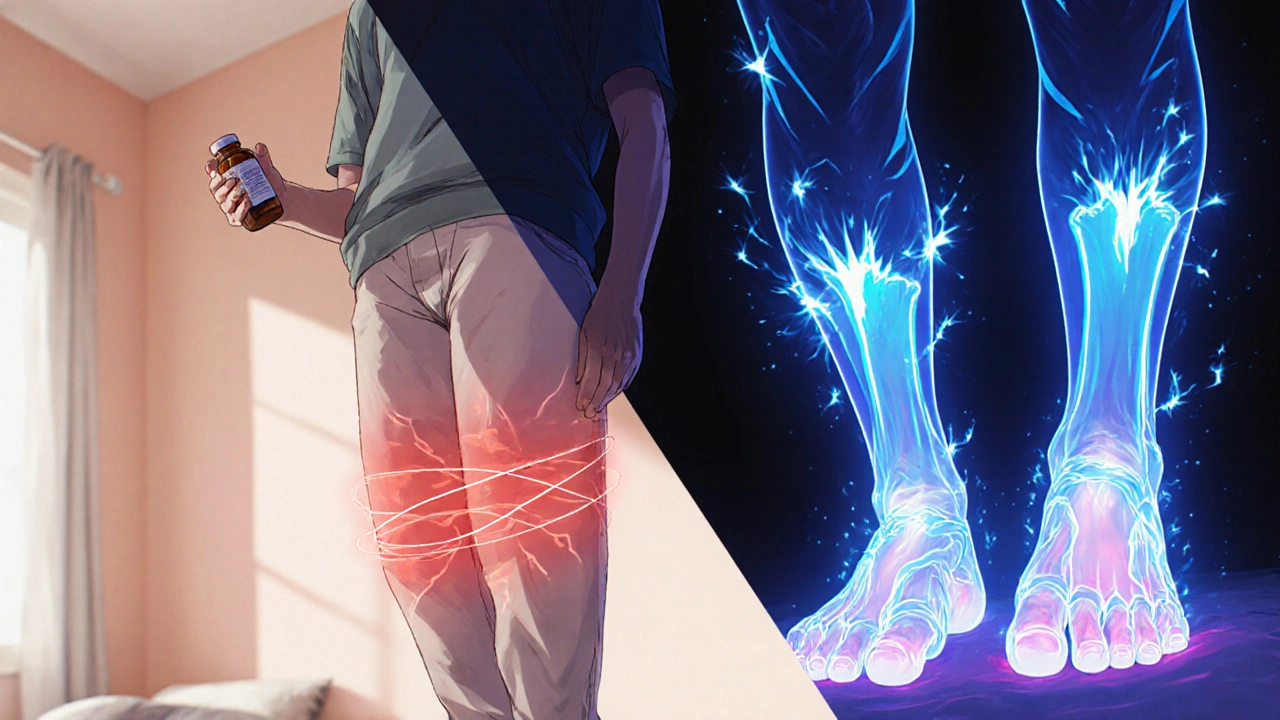
Myopathy shows up as bilateral, proximal muscle weakness - think trouble climbing stairs or lifting groceries. Pain is usually dull, not inflamed, and creatine kinase (CK) may be normal or modestly raised (often < 4 × ULN). The European Atherosclerosis Society (EAS) defines true myopathy when CK surpasses that threshold, occurring in roughly 0.05 % of patients per year.
Key diagnostic clues:
- Symptoms start after statin initiation and improve within 2‑3 weeks of stopping.
- Rechallenge with the same or a different statin reproduces the pain in about 60 % of cases.
- Elevated CK (>4 × ULN) supports a muscular origin.
Risk factors include age > 65, female sex (2:1 ratio), drug interactions (e.g., fibrates), and genetics - notably the HLA‑DRB1*11:01 haplotype and the SLCO1B1 variant, which can raise simvastatin‑induced myopathy risk 4.5‑fold.
Statin‑Related Peripheral Neuropathy: When Nerves Cry Out
Neuropathy feels very different. Patients describe burning, tingling, or numbness that starts in the toes and moves up a “stocking‑glove” pattern. CK stays normal because the muscle itself isn’t damaged. Electrophysiology - nerve conduction studies - typically reveals reduced sensory nerve action potentials, confirming an axonal process.
Evidence is mixed. Some cohort studies link long‑term statin use (>1 year) to higher neuropathy rates, while a 2019 case‑control trial (Warendorf) found a protective odds ratio of 0.5. Proposed mechanisms involve cholesterol‑dependent membrane integrity, vitamin E depletion (LDL carries vitamin E), and CoQ10 shortage affecting neuronal energy metabolism.

Side‑by‑Side Comparison: Myopathy vs Neuropathy
| Feature | Myopathy (Muscle Origin) | Neuropathy (Nerve Origin) |
|---|---|---|
| Typical Distribution | Proximal (hips, thighs, shoulders) | Distal (feet, hands) |
| Primary Symptom | Weakness & aching | Paresthesia, burning, numbness |
| CK Level | Elevated (>4 × ULN in true myopathy) | Normal |
| Electrodiagnostic Findings | Normal EMG or mild myopathic changes | Reduced sensory nerve action potentials; axonal loss |
| Response to Statin Withdrawal | Improvement in 2‑3 weeks; recurrence on rechallenge | Variable; may persist if nerve damage is established |
| Common Risk Factors | Age, female sex, drug interactions, HLA‑DRB1*11:01, SLCO1B1 | Long‑term use, vitamin E deficiency, cholesterol depletion |
Step‑by‑Step Diagnostic Approach
- History taking: note statin start date, dose changes, and symptom timeline.
- Physical exam: assess proximal strength (e.g., hip flexion) and distal sensation (pinprick, vibration).
- Lab work: order CK, thyroid panel, vitamin B12, and fasting glucose to rule out mimickers.
- Statin pause: stop statin for 2‑4 weeks; monitor symptom change.
- If symptoms resolve, suspect myopathy.
- If they linger, move to neuro‑evaluation.
- Electrodiagnostic testing (nerve conduction study & EMG) when neuropathy is a possibility.
- Rechallenge strategy (if needed): switch to a hydrophilic statin (pravastatin or rosuvastatin) at a low dose and titrate slowly.
This algorithm mirrors the Cleveland Clinic’s 2011 recommendation and helps avoid premature labeling of a patient as “statin‑intolerant.”
Management Tailored to the Diagnosis
If myopathy is confirmed:
- Consider a once‑daily, low‑dose, hydrophilic statin.
- Add non‑statin lipid‑lowering agents - ezetimibe, PCSK9 inhibitors, or bile‑acid sequestrants - to meet LDL targets.
- Coenzyme Q10 supplementation has mixed evidence; a 2015 JAMA trial showed no clear benefit, but clinicians sometimes try a trial of 100‑200 mg/day.
- Educate patients about the reversible nature of symptoms when the drug is stopped.
If neuropathy is the culprit:
- Rule out diabetes, alcohol use, vitamin deficiencies, and medication toxicities before blaming the statin.
- If the statin is the only plausible trigger, a gradual taper (e.g., 5 mg reduction per week) may help.
- Consider switching to a statin with less impact on cholesterol‑dependent nerve membranes (e.g., pitavastatin).
- Adjunctive therapies - vitamin E (400 IU/day) and targeted neuropathic pain meds (gabapentin, duloxetine) - can improve quality of life.
When to Call in a Neurologist
If cramps persist beyond 3 months after statin discontinuation, or if EMG shows an axonal pattern, a neuro consult is warranted. The neurologist can differentiate an underlying polyneuropathy that may have been unmasked by statin therapy from a direct drug effect.
Future Directions: Genetics and Personalized Therapy
Research is zeroing in on genetic markers that predict intolerance. The SLCO1B1 *5 variant, for example, raises simvastatin exposure dramatically, prompting dose adjustments before symptoms arise. Ongoing trials like STRENGTH are testing combination therapies (omega‑3 fatty acids with statins) to see if they lower muscle‑related side effects while preserving cardiovascular benefit.
Key Takeaways for Patients and Clinicians
- Distinguish proximal weakness (myopathy) from distal tingling (neuropathy).
- Check CK - high levels point toward muscle injury.
- Use a statin holiday to see if symptoms improve.
- Electrodiagnostic tests are gold standard for neuropathy.
- Tailor therapy: switch statin type, lower dose, or add non‑statin agents.
- Genetic testing may soon guide statin choice for high‑risk patients.
Can I stay on a statin if I have mild muscle cramps?
Yes, most mild cramps are not true myopathy. A low‑dose, hydrophilic statin combined with lifestyle changes often resolves symptoms while preserving heart protection.
What CK level indicates statin myopathy?
The EAS consensus flags CK >4 × the upper limit of normal as true myopathy. Levels below that can still cause discomfort but are usually classified as “statin‑associated muscle symptoms.”
Is statin‑induced neuropathy proven?
Evidence is mixed. Some studies suggest a link, while others show a protective effect. Neuropathy is considered a ‘probable’ side effect, so clinicians must rule out more common causes first.
Should I get genetic testing before starting a statin?
Routine testing isn’t standard yet, but if you have a family history of statin intolerance, a SLCO1B1 test can guide dose selection and reduce myopathy risk.
How long does it take for symptoms to improve after stopping a statin?
Most patients notice relief within 2‑3 weeks. If pain persists beyond a month, consider further evaluation for neuropathy or other muscle disorders.









Kester Strahan
October 24, 2025 AT 16:43 PMStatin‑induced myopathy and neuropathy are two distinct pathophysiological entities, yet they’re often conflated in clin‑care discussions. The myopathic phenotype typically manifests as proximal, bilateral weakness with CK elevation beyond the 4× ULN threshold, while neuropathic presentations involve distal paresthesia without CK perturbation. When you’re evaluating a patient who’s on a high‑potency lipophilic statin like simvastatin, the SLCO1B1*5 allele can boost systemic exposure by up to 200 %, thereby predisposing to myopathy. Conversely, cholesterol depletion in neuronal membranes may underlie the “stocking‑glove” sensory pattern seen with long‑term statin use. A practical algorithm starts with a statin holiday of 2‑4 weeks; if CK normalizes and symptoms abate, myopathy is the likely culprit. If symptoms persist, a nerve conduction study should be pursued to assess for axonal loss. Hydrophilic statins such as pravastatin or rosuvastatin generally present a lower myopathic risk profile, but they’re not exempt from neuropathic side‑effects. Adjunctive CoQ10 supplementation remains controversial, yet many clinicians trial 100‑200 mg daily for its mitochondrial support. Ultimately, personalized therapy guided by pharmacogenomics-particularly SLCO1B1 and HLA‑DRB1*11:01 genotyping-can mitigate adverse events while preserving LDL‑lowering efficacy.
Doreen Collins
October 27, 2025 AT 22:20 PMGlad you found the breakdown helpful, keep listening to your body.
HILDA GONZALEZ SARAVIA
October 31, 2025 AT 09:40 AMWhen patients present with unexplained muscle cramps while on statins, the differential diagnosis must extend beyond the superficial “just a side effect” label. First, a thorough medication history should be taken, noting the exact statin, dose, and duration of therapy, because dose‑dependent toxicity is well‑documented. Second, clinicians should assess for proximal strength deficits by testing hip flexion, knee extension, and shoulder abduction, as these are hallmark signs of myopathy. A normal creatine kinase does not rule out muscle discomfort, but an elevation above four times the upper limit of normal strongly points toward true myopathic injury. Third, the temporal relationship is crucial: symptoms that arise within weeks of initiation and improve upon a statin holiday are classic for SAMS. If the pain persists beyond a month after discontinuation, neuropathic mechanisms become more plausible, especially if the patient reports burning or tingling in a stocking‑glove distribution. Electrophysiological studies, including nerve conduction velocity and EMG, can differentiate axonal loss from myopathic changes. Genetic testing for SLIO1B1 and HLA‑DRB1*11:01 variants can pre‑emptively identify patients at heightened risk for statin‑induced myopathy, allowing dose adjustments before symptoms develop. Vitamin D and CoQ10 levels should be checked, as deficiencies may exacerbate muscle pain, although randomized trials yield mixed results on supplementation benefits. For neuropathy, ruling out diabetes, alcohol use, and B12 deficiency is mandatory before attributing causality to the statin. If a statin‑related neuropathy is suspected, switching to a less lipophilic agent such as pitavastatin or implementing a slow taper can mitigate symptoms. Adjunct therapies like high‑dose vitamin E or duloxetine may provide symptomatic relief, but they should be used in conjunction with a comprehensive work‑up. Importantly, patients should never be labeled “statin intolerant” without exhausting these diagnostic steps, as premature discontinuation can increase cardiovascular risk. Shared decision‑making empowers patients to weigh the modest increase in muscle discomfort against the proven reduction in myocardial infarction risk. Ongoing trials, such as STRENGTH, are evaluating whether adding omega‑3 fatty acids can reduce SAMS while preserving lipid‑lowering efficacy. Until robust data emerge, clinicians must rely on a systematic, individualized algorithm to navigate the myopathy versus neuropathy conundrum.
Lindy Hadebe
November 3, 2025 AT 21:00 PMHonestly, most of these “statin neuropathy” cases are just hype; the literature is riddled with confounding variables and weak associations. If you’re looking for solid evidence, you’ll be disappointed.
Michelle Capes
November 7, 2025 AT 08:20 AMi totally get the frustration 😊, but many patients really do feel those tingling sensations and deserve a compassionate ear.
Dason Avery
November 10, 2025 AT 19:40 PMWhat a beautiful reminder that medicine is both art and science – we must balance hard data with the lived experience of our patients 😌.
Casey Morris
November 14, 2025 AT 07:00 AMIndeed, the nuanced interplay between lipid‑lowering pharmacodynamics and peripheral neurophysiology warrants a multidisciplinary discourse; however, one must remain circumspect when extrapolating cohort findings to individual therapeutics.
Teya Arisa
November 17, 2025 AT 18:20 PMWe appreciate the comprehensive synthesis presented herein; such meticulous delineation of myopathic versus neuropathic mechanisms assists clinicians in making evidence‑based decisions. 😊
Marilyn Pientka
November 21, 2025 AT 05:40 AMIt is ethically indefensible to prescribe statins without pre‑emptively screening for SLCO1B1 polymorphisms; failure to do so constitutes negligent practice and exposes patients to avoidable iatrogenic harm.
Jordan Levine
November 24, 2025 AT 17:00 PMEnough with the bland medical mumbo‑jumbo – statins are killing vibes! 🔥